Bayer to Halt Sales of Essure Birth Control Device by Year’s End
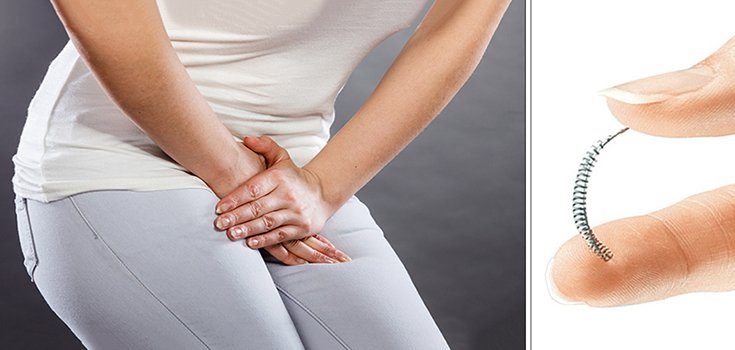
Pharmaceutical giant Bayer announced July 20 that it would stop selling its controversial birth control device Essure in the United States by December 31, 2018. Thousands of women claim to have been injured by the device, including reports of persistent pain, and perforations of the uterus and fallopian tubes. [1]
Essure is the only form of non-surgical permanent birth control available in the United States. The device is inserted into a woman’s fallopian tubes, causing scar tissue to form, which blocks sperm from reaching and fertilizing an egg.
In a statement, U.S. Food and Drug Administration (FDA) Commissioner Scott Gottlieb said:
“The device has been associated with serious risks including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen.”
The FDA has been discussing the safety risks of Essure and what to do about them for years. Bayer allegedly never informed the FDA of the adverse events the device could cause when the agency approved it in 2002. In 2015, the FDA was asked to consider further clinical studies on Essure and to consider making changes to the birth control device’s warning label.
By that time, more than 17,000 women had taken to Facebook to warn others about injuries they had suffered after being implanted with Essure. The vast majority of the women were calling on the FDA to ban Essure outright.
Those women, and others who were injured by Essure, were dismayed when, in 2016, the FDA agreed to add a “black box” warning – the agency’s strongest warning – to Essure’s label, but stopped short of recalling the dangerous device.
In April 2017, the FDA ordered a “patient safety action,” restricting the sale and distribution of Essure. Gottlieb said Bayer complied with the action.
Approximately 750,000 patients worldwide have been used Essure since its 2002 approval, according to FDA estimates.
From 2002-2017, the most commonly reported complaints associated with Essure were:
- Pain/abdominal pain (21,215)
- Heavier menstrual periods/menstrual irregularities (9,846)
- Headache (7,231)
- Fatigue (5,842)
- Weight fluctuations (4,970)
In a statement, Bayer said it was ending sales of Essure because there wasn’t a big enough market for the device anymore. [2]
“This decision is based on a decline in U.S. sales of Essure in recent years and the conclusion that the Essure business is no longer sustainable.”
The company continues to stand by the safety of Essure and says that women currently implanted with the device can continue to “confidently” rely on it.
Bayer suspended sales of Essure outside of the U.S. in September 2017, “citing commercial reasons.”
In announcing it would halt sales of Essure in the U.S. by year’s end, Bayer attributed the decision to a decline in the number of women using permanent contraception; an increase in the use of long-acting, reversible contraceptives, including intrauterine devices, as well as “inaccurate and misleading publicity.”
Bayer makes no mention of the thousands of injury reports, of course, but it’s important to point out that the company is facing more than 16,000 lawsuits filed on behalf of women who allege that Bayer failed to warn patients of the potentially severe complications. [3]
Sources:
[1] CNN
[3] ConsumerSafety
